What are Coupling Agents and their Basic Function
In the coatings, inks, and adhesives industries, do you often encounter these challenges: coatings on glass substrates peeling off after boiling, a sharp drop in adhesive strength on copper or silver products after thermal aging, or uneven dispersion when liquid silanes are added to powder coatings?
These issues, which may appear to be cases of "material incompatibility," often trace back to a key additive—the coupling agent. Many perceive it simply as something that "makes things stick better," but how does it actually "bridge" at the molecular level? How should it be selected for different systems, and what are the hidden pitfalls in its application?
So, what exactly is a coupling agent? A coupling agent is a "molecular bridge" capable of reacting with surface functional groups on inorganic materials (such as metals, glass, or fillers) while also forming chemical bonds or molecular entanglements with organic polymers (like resins or rubbers). Its core function is to resolve the fundamental conflict of "inorganic-organic interface incompatibility."
Detailed Breakdown: The "Dual-Function" Design of Coupling Agents
To understand coupling agents, we must first recognize the "opponents" they address—the inherent opposition between inorganic materials and organic polymers:
Inorganic materials (metals, glass, talc, fiberglass, etc.): Highly polar, with high surface energy; surfaces often feature hydroxyl groups (-OH) or vacant orbitals (e.g., d-orbitals in transition metals).
Organic polymers (epoxy resins, PU, acrylic resins, PP, etc.): Weakly polar, with flexible molecular chains; mostly non-polar or weakly polar structures, making stable bonding with inorganic materials difficult.
The structural design of coupling agents is tailored to "grab both ends," featuring "dual-functional" terminals.
One End "Anchors" the Inorganic Phase: Chemical Bonding with Inorganic Surfaces
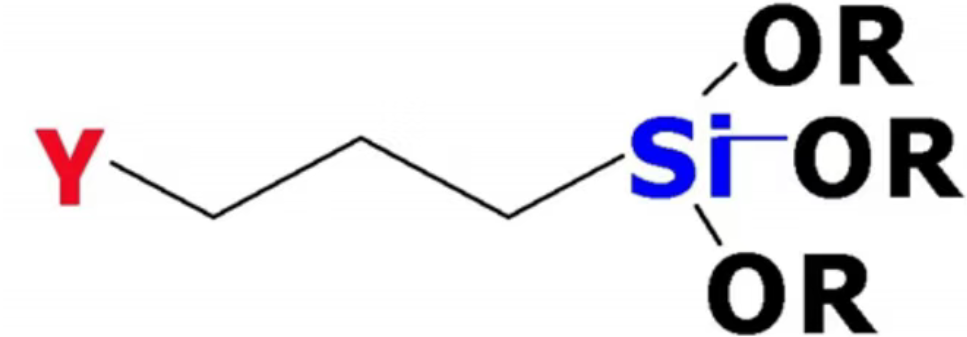
Taking the commonly used silane coupling agents as an example, their inorganic end typically consists of hydrolysable alkoxy groups (-Si-OR, where R is methyl, ethyl, etc.):
Hydrolysis: In the presence of water or moisture, -Si-OR hydrolyzes to form silanol groups (-Si-OH).
Condensation: The silanol groups undergo dehydration condensation with hydroxyl groups on the inorganic material's surface (e.g., -Si-OH on glass, -M-OH on metal oxides), forming strong covalent bonds (-Si-O-Si- or -Si-O-M-). This effectively "nails" the coupling agent to the inorganic surface.
Metal-chelating silanes take this a step further: addressing the challenge of low hydroxyl group presence on surfaces like copper, silver, or nickel, the heterocyclic structures in their molecules (containing atoms like nitrogen or sulfur) can form "coordination bonds" with vacant metal orbitals. They may even create stable five or six-membered "chelating structures"—these bonds are stronger than typical covalent bonds, overcoming the industry challenge of poor adhesion of traditional silanes to copper substrates.
The Other End "Integrates" into the Organic Phase: Stable Bonding with the Resin
The organic end of the coupling agent carries functional groups designed to react with the resin, tailored to the specific resin type:
Epoxy systems: Equipped with epoxy groups, they can directly participate in the curing and cross-linking of epoxy resins.
UV systems: Featuring double bonds, they can react under UV light with free radical or cationic systems.
PU systems: With amino or isocyanate groups, they can react with isocyanate (NCO) to form urea linkages.
Thermoplastic systems (PP/PE): Incorporating long alkyl chains or maleic anhydride groups, they bond with the resin through molecular entanglement (e.g., titanate coupling agents).
Coupling Agent ≠ Surfactant ≠ Dispersant
These three types of additives are often confused, but the key difference lies in whether they form chemical bonds:
Surfactant: Improves interfacial wettability through hydrophilic-lipophilic groups; no chemical bonds are formed, making it prone to migration and failure.
Dispersant: Prevents filler agglomeration via charge repulsion or steric hindrance; primarily relies on physical interactions.
Coupling Agent: Forms chemical bonds connecting both the inorganic and organic phases, acting as a "permanent" interfacial bridge. It not only disperses fillers but also enhances interfacial bonding strength and durability.
Check web pages for more products. For more detail, please contact us.
Post time: Nov-24-2025